Objective & Aims of Project
Exposure of human tissues or cells to reduced oxygen concentration (hypoxia) is a situation encountered both during physiological and pathological processes including embryogenesis, development, pulmonary dysfunction, ischemias, inflammation and cancer. Cells or organisms exposed to hypoxia respond by changing the expression of a great number of genes in order to adapt and survive. Essential to this response are the hypoxia-inducible factors HIF-1 and HIF-2 that regulate the transcription of most hypoxia-target genes, such as those involved in angiogenesis, metabolism, erythropoiesis, cell survival/apoptosis, migration and other tissue specific functions including inflammation and fibrosis.
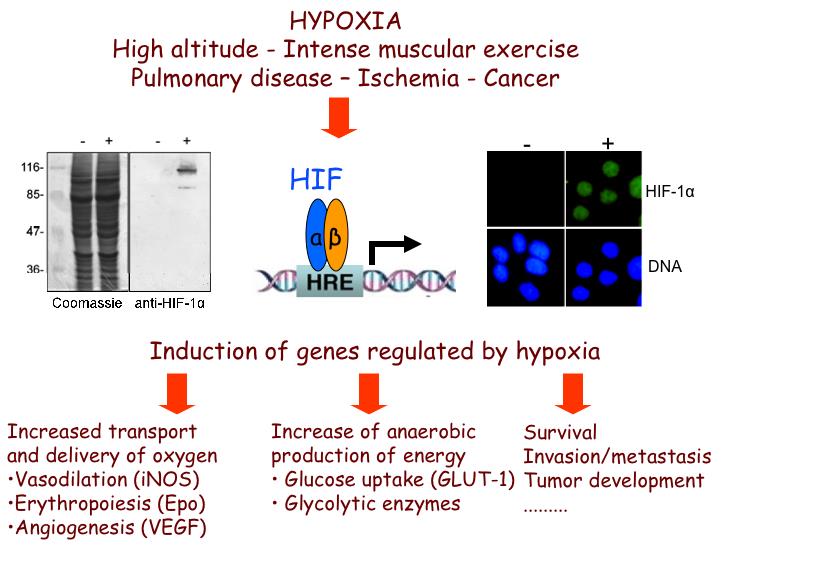
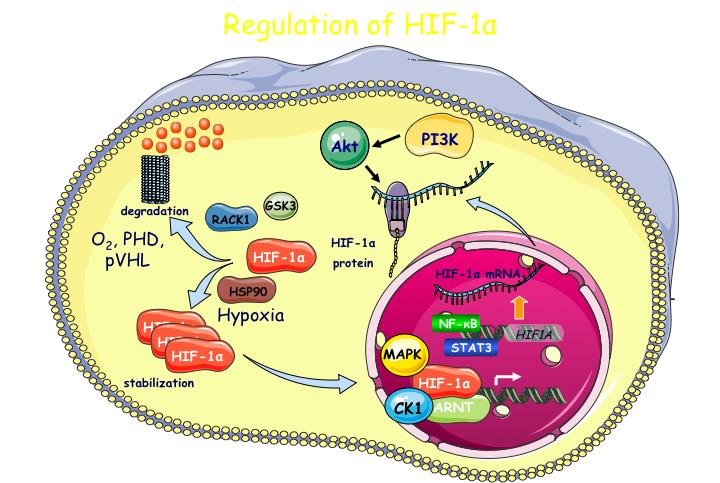
Oxygen levels govern the activity of HIFs through hydroxylation of their regulatory alpha subunits (HIFα). However, tissue-specific, differential or isoform-specific and spatio-temporal control of HIFs in cancer cells also involves oxygen-independent molecular mechanisms, understanding of which requires further investigation and is, therefore, the subject of the first objective of this project.
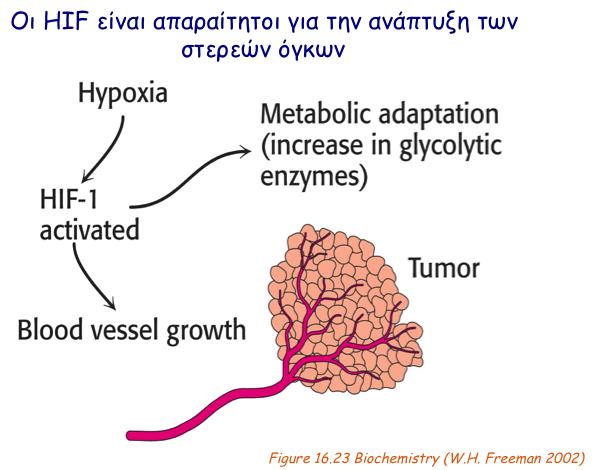
In relation to cancer, a common aspect of many solid tumors is the development of hypoxic regions due to increased cell proliferation and limited blood supply. Hypoxia can promote tumor progression by facilitating angiogenesis and metabolic adaptation of cancer cells. As HIFs are key components of cancer cell adaptation to oxygen deprivation, their alpha subunits are over-expressed in many cancers, are associated with poor patient prognosis and, therefore, represent attractive therapeutic targets. Developing novel peptide agents that can kill cancer cells by specifically targeting HIFs is the second objective of the present project.
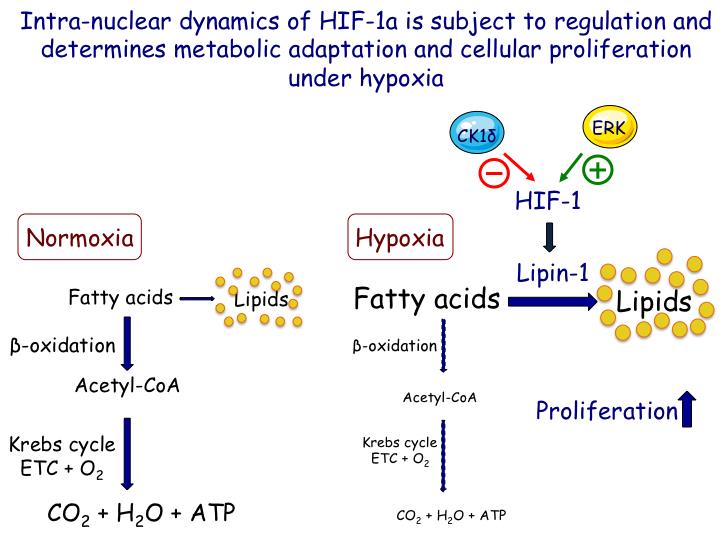
HIFs are also induced by pro-inflammatory agents and are probably implicated in tissue remodelling and fibrosis in chronic inflammatory disorders. Recent studies with transgenic mice additionally suggest that activation of HIF signalling can cause fat accumulation in the liver and steatohepatitis that contribute to metabolic disorders, cirrhosis and hepato-cellular carcinoma (HCC). Hepatocytes express both HIF-1α and HIF-2α but their distinct roles in liver metabolism and pathology are largely unknown and their investigation is, therefore, the subject of the third objective of this project.
As HIFs are involved in many pathological conditions, understanding the common and distinct mechanisms governing regulation and function of HIF-1 and HIF-2 is of great biomedical importance and can lead to the development of novel therapeutic applications.
In brief, the current project aims at understanding the oxygen-independent regulation of HIF-1 and HIF-2 and developing molecular methods to explore and interfere with their function, especially in the context of liver cell metabolism, inflammation and carcinogenesis. The expected results will fulfil the following three objectives:
Objective 1: Elucidation of the role of phosphorylation and protein-protein interactions in the temporal, spatial and differential regulation of HIF isoform activation and function in cancer cells.
Objective 2: Development of specific HIF inhibitors for use in novel anti-cancer and anti-inflammatory treatment strategies.
Objective 3: Delineation of the role of HIF isoforms in liver lipid metabolism and inflammation.


