Objective & Aims of Project
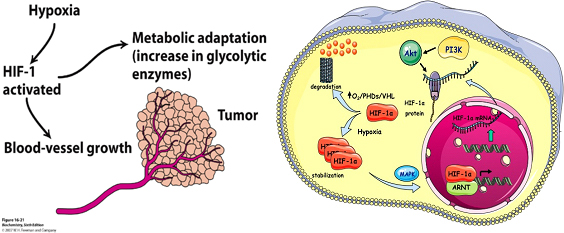
Exposure of human tissues or cells to reduced oxygen concentration (hypoxia) is a situation encountered both during physiological and pathological processes including embryogenesis, development, pulmonary dysfunction, ischemias, inflammation and cancer. Cells or organisms exposed to hypoxia respond by changing the expression of a great number of genes in order to adapt and survive. Essential to this response are the hypoxia-inducible factors HIF-1 and HIF-2 that regulate the transcription of most hypoxia-target genes, such as those involved in angiogenesis, metabolism, erythropoiesis, cell survival/apoptosis, migration and other tissue specific functions including inflammation and fibrosis.
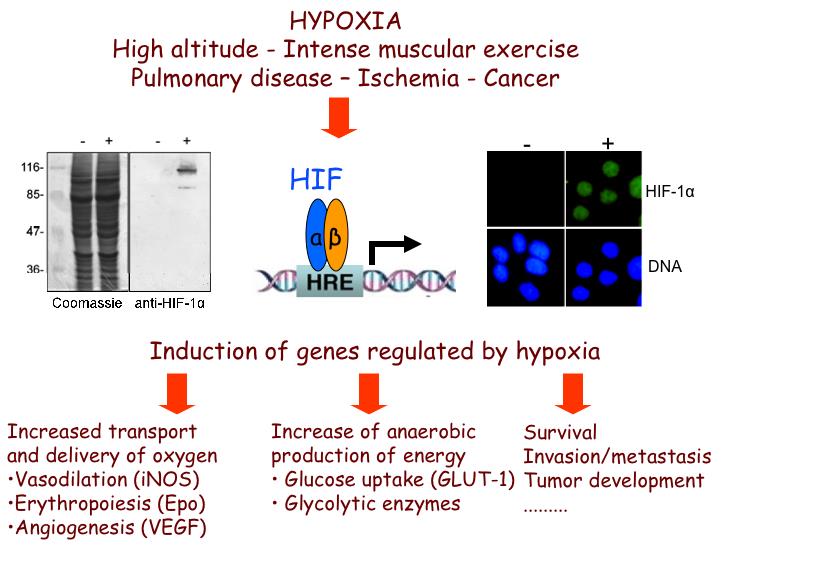
Oxygen levels govern the activity of HIFs through hydroxylation of their regulatory alpha subunits (HIFα). However, tissue-specific, differential or isoform-specific and spatio-temporal control of HIFs in cancer cells also involves oxygen-independent molecular mechanisms, understanding of which requires further investigation and is, therefore, the subject of the first objective of this project.
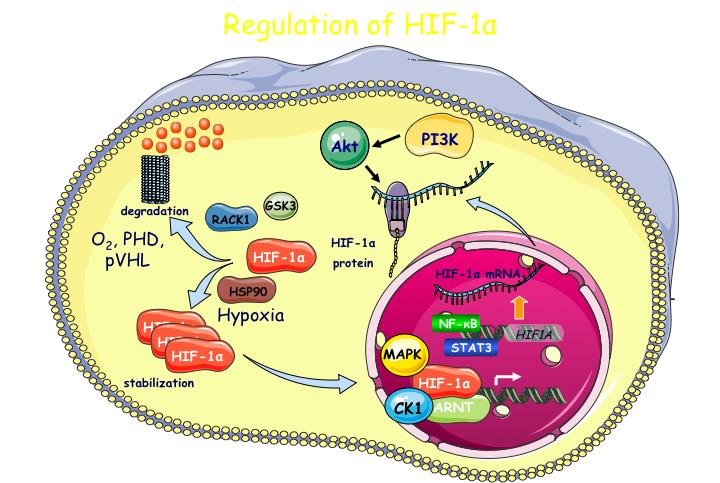
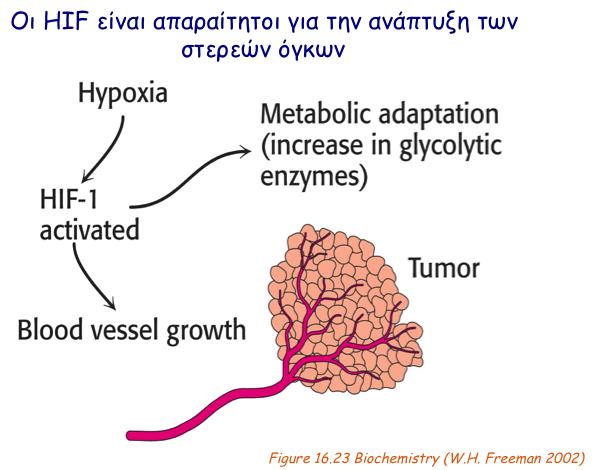
In relation to cancer, a common aspect of many solid tumors is the development of hypoxic regions due to increased cell proliferation and limited blood supply. Hypoxia can promote tumor progression by facilitating angiogenesis and metabolic adaptation of cancer cells. As HIFs are key components of cancer cell adaptation to oxygen deprivation, their alpha subunits are over-expressed in many cancers, are associated with poor patient prognosis and, therefore, represent attractive therapeutic targets. Developing novel peptide agents that can kill cancer cells by specifically targeting HIFs is the second objective of the present project.
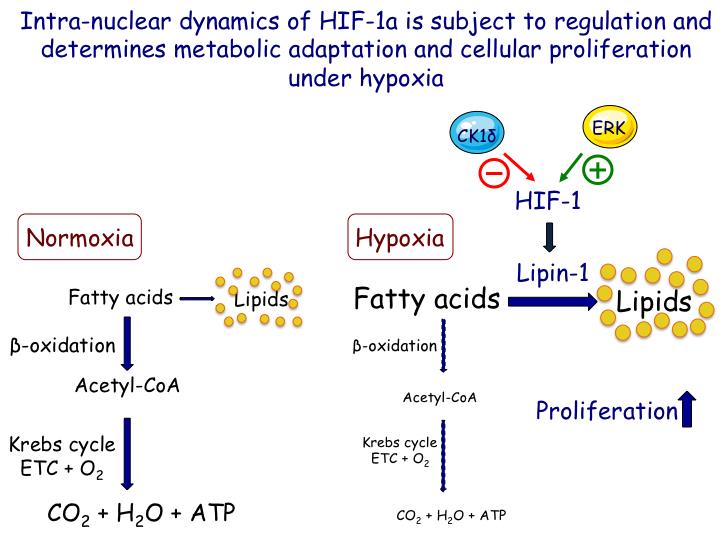
HIFs are also induced by pro-inflammatory agents and are probably implicated in tissue remodelling and fibrosis in chronic inflammatory disorders. Recent studies with transgenic mice additionally suggest that activation of HIF signalling can cause fat accumulation in the liver and steatohepatitis that contribute to metabolic disorders, cirrhosis and hepato-cellular carcinoma (HCC). Hepatocytes express both HIF-1α and HIF-2α but their distinct roles in liver metabolism and pathology are largely unknown and their investigation is, therefore, the subject of the third objective of this project.
As HIFs are involved in many pathological conditions, understanding the common and distinct mechanisms governing regulation and function of HIF-1 and HIF-2 is of great biomedical importance and can lead to the development of novel therapeutic applications.
In brief, the current project aims at understanding the oxygen-independent regulation of HIF-1 and HIF-2 and developing molecular methods to explore and interfere with their function, especially in the context of liver cell metabolism, inflammation and carcinogenesis. The expected results will fulfil the following three objectives:
Objective 1: Elucidation of the role of phosphorylation and protein-protein interactions in the temporal, spatial and differential regulation of HIF isoform activation and function in cancer cells.
Objective 2: Development of specific HIF inhibitors for use in novel anti-cancer and anti-inflammatory treatment strategies.
Objective 3: Delineation of the role of HIF isoforms in liver lipid metabolism and inflammation.
Publications with project results
Α. Results presented in Greek and International Scientific Conferences
Pangou E., Simos G. and Liakos P. (2014) Pro-inflammatory cytokines TNF-α, IL-6 and IL-1β exhibit differential effects on the expression and transcriptional activity of HIFs in human liver cancer cells. FEBS-EMBO Paris 2014 Congress. The FEBS Journal 281 (S1), p. 156, Abstract SUN-267.
Triantafyllou E.-A., Tsapournioti S., Mylonis I., Simos G., Molyvdas P.-A., Paraskeva E. (2014) The inflammatory mediator tumor necrosis factor α (TNFα) has cell type-dependent effects on hypoxia-inducible factor 1 (HIF-1). FEBS-EMBO Paris 2014 Congress. The FEBS Journal 281 (S1), p. 176, Abstract SUN-328.
Kourti M., Giakoumakis N.-N., Lygerou Z., Simos G. and Mylonis I. (2014) ERK-dependent association of HIF-1a with chromatin via a peptide that can inhibit HIF-1 activity when over-expressed in hepatocellular carcinoma cells. FEBS-EMBO Paris 2014 Congress. The FEBS Journal 281 (S1), p. 462, Abstract TUE-093.
Simos G. (2014) Regulation of nuclear complex formation and transport of HIF-1α and its role in metabolic adaptation of cancer cells to hypoxia. International SEE DRUG Workshop “Views into nuclear function”, 11-13 Σεπτ. 2014, Πάτρα. Abstract Book, p. 15.
Triantafyllou E.-A., Tsapournioti S., Mylonis I., Simos G., Molyvdas P.-A., Paraskeva E. (2014) The effects of tumor necrosis factor α (TNFα) and hypoxia on the induction of nuclear factor-kB (NF-kB), HIF-1 and inflammation marker genes. International SEE DRUG Workshop “Views into nuclear function”, 11-13 Σεπτ. 2014, Πάτρa. Abstract Book, p. 86.
Triantafyllou E.A., Tsapournioti S., Myloni I., Simos G. & E. Paraskeva (2014) Interplay of inflammation and hypoxia on the activation of nuclear factor-κB (NF-κB) and HIF-1 pathways. 65th National Meeting of the Hellenic Society of Biochemistry and Molecular Biology, 28-30 November 2014, Thessaloniki. Abstracts, P091.
Pangou E., Befani C., Mylonis I., Samiotaki M., Panayotou G., Simos G. & P. Liakos (2014) CK1δ directly targets distinct sites in HIF-2α. 65th National Meeting of the Hellenic Society of Biochemistry and Molecular Biology, 28-30 November 2014, Thessaloniki. Abstracts, P096.
Rimenidi G., Kourti M., Simos G. & I. Mylonis (2014) Intracellular delivery of HIF-1α ERK-targeted domain peptides and their effect on HIF-1 activity in hepatocellular carcinoma cells. 65th National Meeting of the Hellenic Society of Biochemistry and Molecular Biology, 28-30 November 2014, Thessaloniki. Abstracts, P133.
Karagiota A., Simos G. & I. Mylonis (2014) Functional analysis of Flag–tagged HIF-1α forms carrying mutations inside their ERK-targeted domain. 65th National Meeting of the Hellenic Society of Biochemistry and Molecular Biology, 28-30 November 2014, Thessaloniki. Abstracts, P134
Befani C., Pangou E., Mylonis I., Simos G. & P. Liakos (2014) ERK1/2 phosphorylates HIF-2α and promotes its transcriptional activity. 65th National Meeting of the Hellenic Society of Biochemistry and Molecular Biology, 28-30 November 2014, Thessaloniki. Abstracts, P142.
Project Information
Title:
Targeting the hypoxia-inducible transcription factors HIFs in inflammation and cancer.
Principal Investigator:
George Simos, Professor of Biochemistry
Host Laboratory:
Laboratory of Biochemistry, Faculty of Medicine, University of Thessaly
Duration and Budget:
18 months (29/1/2014-31/7/2015), 295.000 €
Administrative Agency:
Research Committee, University of Thessaly
Members of Research Group:
George Simos, Professor of Biochemistry
Eleni Georgatsou, Assoc. Professor of Biochemistry & Molecular Biology
Efrosini Paraskeva, Assoc. Professor of Cellular Physiology
Panagiotis Liakos, Assist. Professor of Medical Biochemistry
Ilias Mylonis, Assist. Professor of Biochemistry
Georgia Chachami, Lecturer of Cellular Biochemistry
Emmanouil Venieris, member of Laboratory & Teaching Staff
Eva Paggou, PhD student
Eleni Triantafyllou, PhD student
Aggeliki Karagiota, PhD student

Short Description:
Hypoxia characterizes major pathological processes such as inflammation and cancer and affects gene expression through the hypoxia-inducible transcription factors HIF-1 and HIF-2. Elucidation of the common and isoform- or tissue-specific mechanisms governing HIFs is important to understand the response of cancer cells to hypoxia and can have novel therapeutic applications. We, therefore, investigate the oxygen-independent regulation of HIFs in cancer cells as well as their connection to tissue inflammation and fibrosis by focusing on:
a. The control of HIFs by phosphorylation and protein-protein interactions in cancer cells. We are trying to identify kinases and phosphatases that modify and regulate HIFs and are developing methods for in situ detection of HIF protein complexes using microscopy.
b. The discovery of new anti-cancer and anti-inflammatory targeted treatment strategies based on HIF inhibition. We aim to generate agents that specifically inhibit phosphorylation of HIF-1alpha and explore their efficiency as anticancer agents.
c. The role of HIFs in hepatocyte lipid metabolism and inflammation, two processes linked in steatohepatitis and hepatocarcinoma. We aim to study the expression profile of key lipogenic enzymes under hypoxic conditions and the role of hypoxia and lipogenesis in the production of hepatocyte proinflammatory cytokines and profibrotic mediators.
The results will provide better insight into the involvement of HIFs in cancer cell survival and proliferation and help identify novel avenues for the development of methods useful for managing cancer, inflammation and common metabolic disorders.
Members of Research Group

Eleni Georgatsou,
Assoc. Professor of Biochemistry & Molecular Biology


Panagiotis Liakos,
Assist. Professor of Medical Biochemistry




Eva Paggou,
PhD student

Eleni Triantafyllou,
PhD student

Aggeliki Karagiota,
PhD student

Page 2 of 2


